Diels alder Reaction
The Diels alder reaction combines a dinene (a molicule with two alternating double bonds) and a dienophile (an alkene) to make rings and bicyclic compounds. The three double bonds in the two starting materials are converted into two new single bonds and one new double bond. Since the reaction forms two new carbon-carbon bonds in a single step it is a vary useful and powerful reaction (one which earned otto Diels and Kurt Alder a Noble prize in chemistry for discovering it).
Typically the Diels alder reaction works best when either the diene is substituted with electron donating groups like (OR, -NR, etc) or where the dienophile is substituted with electron withdrawing group like (No2 ,-CN, -COR, etc).
Conformational requirements of the diene
One quirk of the Diels alder reaction is that the diene is required to be in the s-cis conformation in order for the diels alder reaction to work. The s-cis conformation has both of the double bond pointing on the sane side of the carbon carbon single bond that connects them. In solution the carbon carbon single bond in the diene that connects the two alkanes is constantly rotating so at equilibrium there is usually some mixture of dienes the s-trans conformation and some in the s-cis conformation. The onces that are at that moment in the s-trans conformation do not react while the once in the s-cis confirmation can go on the react
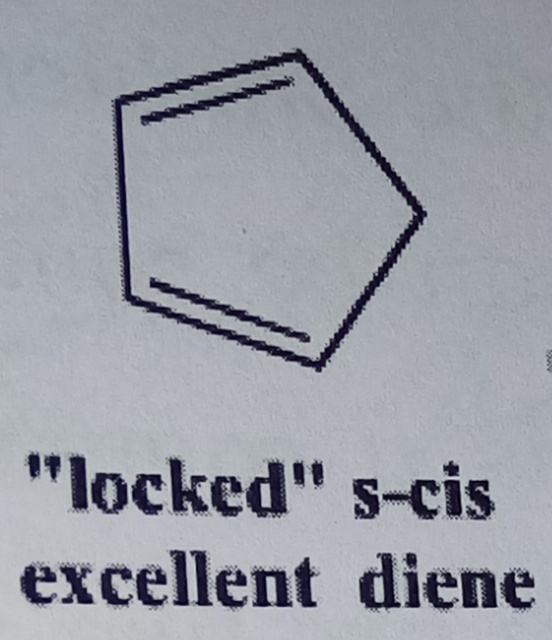
Because of the Dieels alder's requirement for having tje diene in a s-cis confirmation, dienes in rings react particularly rapidly because they are locked in the s-cis confirmation. Unlike dienes in open chains in which there is usually some proportion of the diene in the interactive s-trans confirmation diene in rings are held in the reactive confirmation at all times by the constraints of the ring making them react faster.
......................................................................................







0 Comments